They were called flipper babies back then. I've seen many. My brother was born with Cerebral Palsy and and was mentally challenged. He went to what was then called a "Handicapped" school. I volunteered there and there were several, also one with flipper legs. Very sad. But very lovable. Sweetest dispositions considering their physical limitations, they had the I Can Do It attitude.In America women were given the drug. I met a thalidomide man when I was a teenager. He was hanging out with us. He had legs but no arms and his hands grew out of his shoulders.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Fully Vaccinated ........Covid ....
- Thread starter charry
- Start date
In July 1962, the Food and Drug Administration sent an urgent message to its field offices with an assignment it said was “one of the most important we have had in a long time.”If it was given, it was given illegally as the FDA rejected its use, by this individual... Frances Oldham Kelsey.
Quit making baseless claims.
Overseas, thousands of babies in Germany, England and other countries were being born with severe defects tied to their mothers’ use of thalidomide, a drug widely taken for insomnia, morning sickness and other ailments.
Meanwhile, the federal government sought to figure out what had happened in the United States, and how many babies had been affected.
The drug was not approved in the United States in the 1960s, but as many as 20,000 Americans were given thalidomide in the 1950s and 1960s as part of two clinical trials operated by the American drug makers Richardson-Merrell and Smith, Kline & French.
https://www.nytimes.com/2020/03/23/health/thalidomide-fda-documents.html
JimBob1952
Senior Member
IMO it doesn't, matter who said it, a Dr or "expert" with dozens of titles behind their name or a garbage collector with a sh*t title, the true statement shows something is very wrong.
Ok, I'm not a doctor, but I play one in my own imagination. And I'm making the "true statement" that the Pfizer and Moderna vaccines are safe and effective, although, like the flu vaccine, their effectiveness wanes after a number of months. Just got my booster this morning.
I don't understand the anti-vaxxers, but we all live in our own worlds.
RadishRose
SF VIP
- Location
- Connecticut, USA
You don't have to be so nasty, Harry.Deflection alert!! (how sad!!)
JimBob1952
Senior Member
In July 1962, the Food and Drug Administration sent an urgent message to its field offices with an assignment it said was “one of the most important we have had in a long time.”
Overseas, thousands of babies in Germany, England and other countries were being born with severe defects tied to their mothers’ use of thalidomide, a drug widely taken for insomnia, morning sickness and other ailments.
Meanwhile, the federal government sought to figure out what had happened in the United States, and how many babies had been affected.
The drug was not approved in the United States in the 1960s, but as many as 20,000 Americans were given thalidomide in the 1950s and 1960s as part of two clinical trials operated by the American drug makers Richardson-Merrell and Smith, Kline & French.
https://www.nytimes.com/2020/03/23/health/thalidomide-fda-documents.html
That was about 60 years ago. You don't suppose science and medicine might have progressed somewhat in the years since then?
Anyone posing as a doctor, who is that ignorant about spelling, has just put himself in jail. No need for a cop. And all the rest of his whining is equally dumb. He's perfectly free to kill himself, but I'd hate to think he is actually influencing people to believe him.
Sometimes, the spelling and grammar are enough to reveal who the phonies are.
My IT folks say spelling and grammar errors are one among the first things to look for when you are sent a suspicious email.
Ok, I'm not a doctor, but I play one in my own imagination. And I'm making the "true statement" that the Pfizer and Moderna vaccines are safe and effective, although, like the flu vaccine, their effectiveness wanes after a number of months. Just got my booster this morning.
I don't understand the anti-vaxxers, but we all live in our own worlds.
Good for you, you believe in the vaccine and did what you felt was right for you. Just as those not believing its safe are doing what's best for them.
I'm not anti vaccine I've had every vaccination available until this one.
My comment was in response to this one,That was about 60 years ago. You don't suppose science and medicine might have progressed somewhat in the years since then?
My IT folks say spelling and grammar errors are one among the first things to look for when you are sent a suspicious email.
"Harry Le Hermit said:
If it was given, it was given illegally as the FDA rejected its use, by this individual... Frances Oldham Kelsey.
Quit making baseless claims."
chic did not make a "baseless claim"
JimBob1952
Senior Member
Good for you, you believe in the vaccine and did what you felt was right for you. Just as those not believing its safe are doing what's best for them.
I'm not anti vaccine I've had every vaccination available until this one.
Those not believing it's safe are doing what they think is best for them. They are also putting the rest of the population at risk. That's a crucial difference.
Maryatrics
New Member
- Location
- United States, Virginia
That is why the vaccinated people have to do everything imaginable to keep them safe and if that is wearing a mask indoors and even outdoors in some occasions then so be it. if that means Social Distancing and staying away from heavily crowded areas than you do just that to keep yourself as safe as you can be. I am not saying keep yourself in a bubble or at home all the time, but be sensible to what you feel is safe around others.Those not believing it's safe are doing what they think is best for them. They are also putting the rest of the population at risk. That's a crucial difference.
JimBob1952
Senior Member
That is why the vaccinated people have to do everything imaginable to keep them safe and if that is wearing a mask indoors and even outdoors in some occasions then so be it. if that means Social Distancing and staying away from heavily crowded areas than you do just that to keep yourself as safe as you can be. I am not saying keep yourself in a bubble or at home all the time, but be sensible to what you feel is safe around others.
For example, don't get in a huddle like Aaron Rogers has been doing....
Maryatrics
New Member
- Location
- United States, Virginia
Those professional athletes are tested daily for Covid. If they are any signs and I am aware that some don't show signs, but if they have signs of Covid or test positive for Covid they are quarantined for I believe 14 days.For example, don't get in a huddle like Aaron Rogers has been doing....
His big crime was lying to his team mates about it.For example, don't get in a huddle like Aaron Rogers has been doing....
Irwin
Well-known Member
- Location
- Denver, Colorado, U.S.A.
He put his teammates at risk, and he also put a lot of other people at risk. Vaccinated people are far less likely to contract the virus and if they do contract it, they are less likely to spread it because they have a lower concentration of it in their breath. He's been speaking at press conferences without wearing a facemask and potentially putting reporters and other people at risk. That's purely narcissistic behavior. He only cares about himself and apparently lacks critical thinking skills when it comes to matters off the field.His big crime was lying to his team mates about it.
Maryatrics
New Member
- Location
- United States, Virginia
Aaron Rodgers has taken a medication called ivermectin which is an Anti-Parasite medication and it is RDA approved for that, but is controversial for Covid-19. I suppose he believes in it and also believes he is allergic to the Covid-19 vaccine in someway.
Warrigal
SF VIP
- Location
- Sydney, Australia
My state, NSW, has succeeded in lowering this last upsurge of Covid.
Vaccination, on top of a number of health mandates like QR check in codes, mass testing centres, wearing masks and social distancing, has made a huge difference.
Below is the daily update report. Note that we are almost at 90% fully vaccinated.
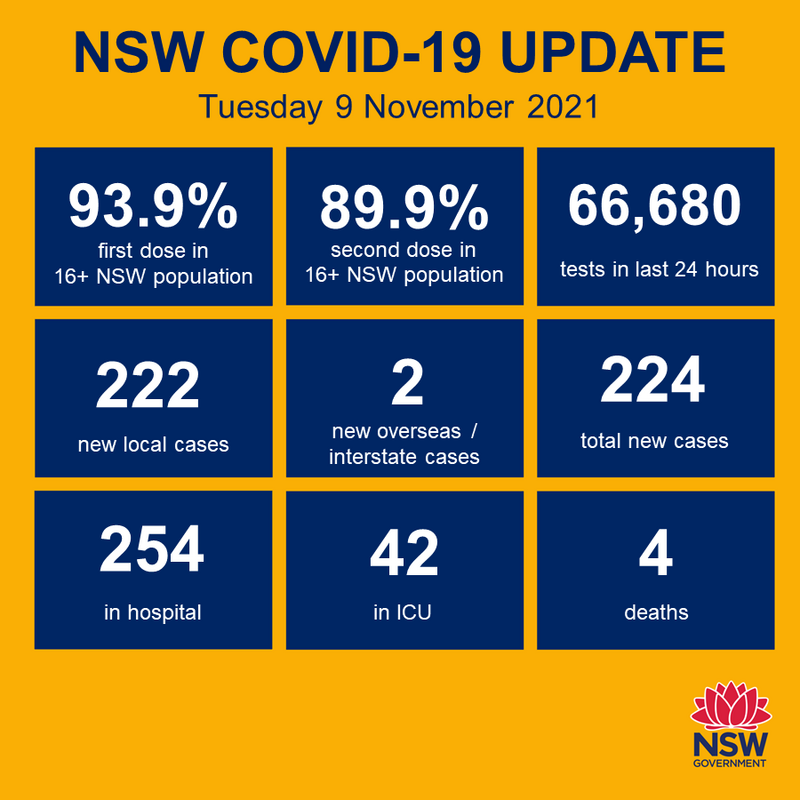
Edit - it was no so long ago that the daily number of cases was in excess of 2,000.
Vaccination, on top of a number of health mandates like QR check in codes, mass testing centres, wearing masks and social distancing, has made a huge difference.
Below is the daily update report. Note that we are almost at 90% fully vaccinated.

Edit - it was no so long ago that the daily number of cases was in excess of 2,000.
Last edited:
Shero
Senior Member
- Location
- Transient in the Land of Oz
.That is why the vaccinated people have to do everything imaginable to keep them safe and if that is wearing a mask indoors and even outdoors in some occasions then so be it. if that means Social Distancing and staying away from heavily crowded areas than you do just that to keep yourself as safe as you can be. I am not saying keep yourself in a bubble or at home all the time, but be sensible to what you feel is safe around others.
The only way to keep the community safe is to be vaccinated!!
Shero
Senior Member
- Location
- Transient in the Land of Oz
It makes them dangerous!!I am not) say it is safe to not do so. Even then it comes down to the decision of the individual if they still want to wear a mask. It still doesn't make them stupid if they decide to do so.
Maryatrics
New Member
- Location
- United States, Virginia
I am 100% in support for being vaccinated and I am fully vaccinated and also will get the booster. The thing is I am also aware that not everyone will be vaccinated..
The only way to keep the community safe is to be vaccinated!!
I do everything in my power to stay safe and that includes wearing a mask indoors. I don't always choose to wear a mask outside unless I am in a crowded area which hardly ever happens. I also attempt to Social Distance like when I am grocery shopping. I feel these things are what I can do to keep myself as safe as possible other than staying at home all the time. I am unaware who else is vaccinated or not so these things keep my mind at ease for me.It makes them dangerous!!
Aneeda72
Well-known Member
The Delta Covid virus is shed for up to 21 days, unfortunatelyThose professional athletes are tested daily for Covid. If they are any signs and I am aware that some don't show signs, but if they have signs of Covid or test positive for Covid they are quarantined for I believe 14 days.
Thanks for sharing Becky. This did exist in the U.S. Women who were prone to miscarriage took this drug to bring their babies to term. It was a very sad result for them.They were called flipper babies back then. I've seen many. My brother was born with Cerebral Palsy and and was mentally challenged. He went to what was then called a "Handicapped" school. I volunteered there and there were several, also one with flipper legs. Very sad. But very lovable. Sweetest dispositions considering their physical limitations, they had the I Can Do It attitude.
Warrigal
SF VIP
- Location
- Sydney, Australia
Yes, we had a thalidomide girl in the secondary school where I was teaching and she was amazing. Her spirit was indominable. Over my years of teaching I have been inspired by the girls who overcame dreadful handicaps.They were called flipper babies back then. I've seen many. My brother was born with Cerebral Palsy and and was mentally challenged. He went to what was then called a "Handicapped" school. I volunteered there and there were several, also one with flipper legs. Very sad. But very lovable. Sweetest dispositions considering their physical limitations, they had the I Can Do It attitude.
The nuns refused to turn away any girl who was from our feeder area and we wrapped ourselves around them to make sure they were included. Be they almost totally blind, or deaf, physically challenged or intellectually limited we brought them in from the cold and made sure they were warmly treated by the staff and other students.
What point is there to religion if it is not put into practice?
I learnt so much about life and love from these kids, and also from the nuns.
Since the vaccine's effectiveness wanes after a number of months, how many months will go by between each booster?Ok, I'm not a doctor, but I play one in my own imagination. And I'm making the "true statement" that the Pfizer and Moderna vaccines are safe and effective, although, like the flu vaccine, their effectiveness wanes after a number of months. Just got my booster this morning.
I don't understand the anti-vaxxers, but we all live in our own worlds.
That's a crock - an attempt to guilt everyone into getting vaccinated.Those not believing it's safe are doing what they think is best for them. They are also putting the rest of the population at risk. That's a crucial difference.
They tried the same BS to sell more flu shots - "If you don't get a flu shot, you're making others sick." Kaiser Permanente hung a big banner over the entrance to their hospital that said that. It only works for the ignorant. If your vaccine worked, you would be protected from the unvaccinated, so NO unvaccinated are putting anyone at risk......unless, of course, the vaccine doesn't work.
CarolfromTX
Senior Member
- Location
- Central Texas
How are they putting the rest of the population at risk, assuming the vaccine is effective? I’m vaccinated. So if someone else is not, how is that a risk to me? I can still get it, you say? But what are the odds? Highly doubtful I’ll die from it, let alone be hospitalized. And life is not without risk. It’s not. It’s the damn mandates I’m against, not the vaccine. And I’m against the insistence of vaccine nazis that everyone must be vaccinated.Those not believing it's safe are doing what they think is best for them. They are also putting the rest of the population at risk. That's a crucial difference.

